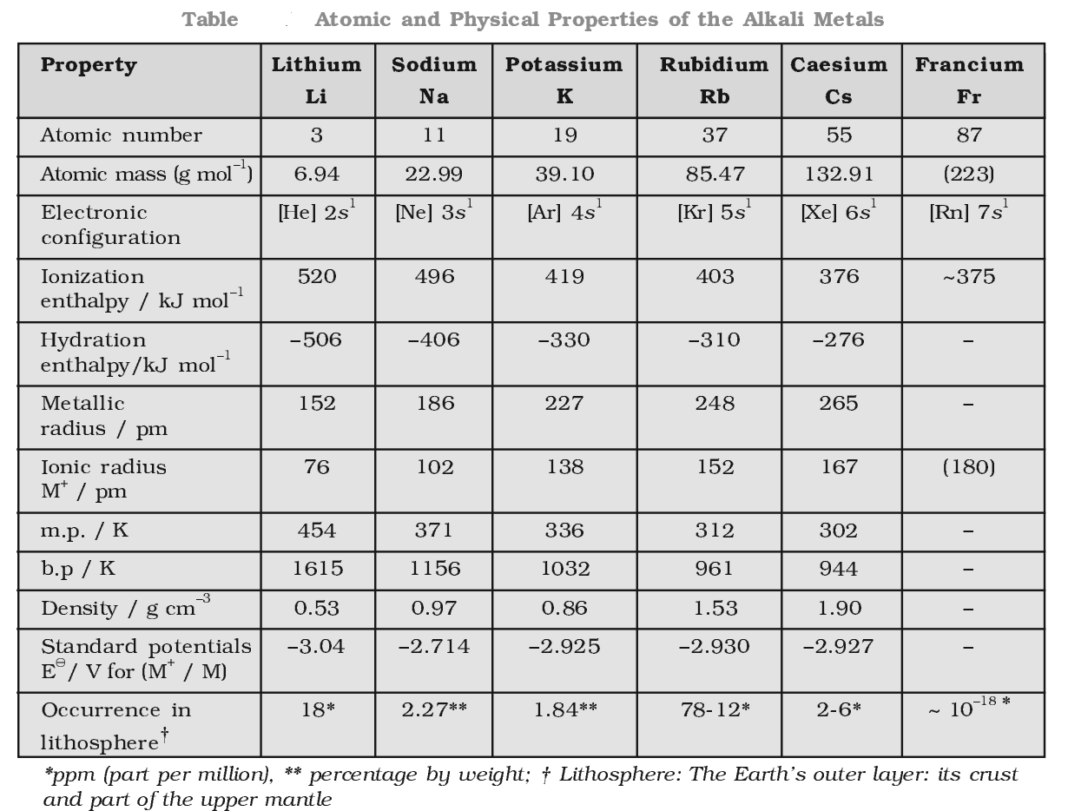
Special Properties Of Alkali Metals . In keeping with overall periodic trends,. the alkali metals are the elements located in group ia of the periodic table. The one outer electron is easily lost, forming the. They react with water to produce an alkaline metal hydroxide solution and hydrogen. the group 1 elements are all soft, reactive metals with low melting points. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). general properties of the alkali metals. The alkali metals are lithium, sodium, potassium, rubidium,. the lone outer shell electrons leads the alkali metal elements to share several common properties: Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the. the alkali metals are a group of elements in the periodic table with similar properties: the alkali metals tend to form +1 cations.
from selfstudypoint.in
the alkali metals are a group of elements in the periodic table with similar properties: They react with water to produce an alkaline metal hydroxide solution and hydrogen. the alkali metals are the elements located in group ia of the periodic table. The alkali metals are lithium, sodium, potassium, rubidium,. In keeping with overall periodic trends,. the group 1 elements are all soft, reactive metals with low melting points. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). the lone outer shell electrons leads the alkali metal elements to share several common properties: the alkali metals tend to form +1 cations.
Group 1 Elements Alkali Metals
Special Properties Of Alkali Metals Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The alkali metals are lithium, sodium, potassium, rubidium,. the alkali metals tend to form +1 cations. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). In keeping with overall periodic trends,. the group 1 elements are all soft, reactive metals with low melting points. the alkali metals are a group of elements in the periodic table with similar properties: The one outer electron is easily lost, forming the. general properties of the alkali metals. They react with water to produce an alkaline metal hydroxide solution and hydrogen. the alkali metals are the elements located in group ia of the periodic table. the lone outer shell electrons leads the alkali metal elements to share several common properties: Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the.
From www.slideshare.net
Physical and chemical properties of alkali metals Special Properties Of Alkali Metals In keeping with overall periodic trends,. the alkali metals tend to form +1 cations. the group 1 elements are all soft, reactive metals with low melting points. general properties of the alkali metals. the alkali metals are the elements located in group ia of the periodic table. Various properties of the group 1 elements are summarized. Special Properties Of Alkali Metals.
From www.slideserve.com
PPT THE PERIODIC TABLE PowerPoint Presentation, free download ID Special Properties Of Alkali Metals the alkali metals tend to form +1 cations. the alkali metals are the elements located in group ia of the periodic table. The one outer electron is easily lost, forming the. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The alkali metals are lithium, sodium, potassium, rubidium,. general properties of the alkali metals.. Special Properties Of Alkali Metals.
From exobgridh.blob.core.windows.net
What Are The Common Physical Properties Of Alkali Metals at Israel Special Properties Of Alkali Metals the alkali metals tend to form +1 cations. general properties of the alkali metals. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The alkali metals are lithium, sodium, potassium, rubidium,.. Special Properties Of Alkali Metals.
From www.pinterest.com
Group 1 (Alkali Metals) Alkali metal, Physical properties, Physics Special Properties Of Alkali Metals the group 1 elements are all soft, reactive metals with low melting points. In keeping with overall periodic trends,. general properties of the alkali metals. the alkali metals are the elements located in group ia of the periodic table. the alkali metals tend to form +1 cations. Cation formation is favored by the relatively low ionization. Special Properties Of Alkali Metals.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation ID5525387 Special Properties Of Alkali Metals the alkali metals are the elements located in group ia of the periodic table. They react with water to produce an alkaline metal hydroxide solution and hydrogen. the lone outer shell electrons leads the alkali metal elements to share several common properties: The alkali metals are lithium, sodium, potassium, rubidium,. general properties of the alkali metals. . Special Properties Of Alkali Metals.
From www.pinterest.com.mx
Alkali Metals Properties Periodic Table iOS app Alkali metal Special Properties Of Alkali Metals They react with water to produce an alkaline metal hydroxide solution and hydrogen. the alkali metals are a group of elements in the periodic table with similar properties: In keeping with overall periodic trends,. The one outer electron is easily lost, forming the. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The alkali metals are. Special Properties Of Alkali Metals.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions Special Properties Of Alkali Metals general properties of the alkali metals. The alkali metals are lithium, sodium, potassium, rubidium,. the alkali metals tend to form +1 cations. The one outer electron is easily lost, forming the. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the. the lone. Special Properties Of Alkali Metals.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic Special Properties Of Alkali Metals the alkali metals tend to form +1 cations. They react with water to produce an alkaline metal hydroxide solution and hydrogen. The one outer electron is easily lost, forming the. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). the alkali metals are the elements located in group ia of the periodic table. In keeping. Special Properties Of Alkali Metals.
From selfstudypoint.in
Group 1 Elements Alkali Metals Special Properties Of Alkali Metals Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the. the alkali metals are a group of elements in the periodic table with similar properties: the alkali metals are the elements located in group ia of the periodic table. Various properties of the group. Special Properties Of Alkali Metals.
From www.chemistry4students.com
Chemistry 4 Students Alkali Metals (group 1 elements) Special Properties Of Alkali Metals general properties of the alkali metals. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the. They react with water to produce an alkaline metal hydroxide solution and hydrogen. the alkali metals are a group of elements in the periodic table with similar properties:. Special Properties Of Alkali Metals.
From www.youtube.com
Chemical Properties of Alkali Metal YouTube Special Properties Of Alkali Metals They react with water to produce an alkaline metal hydroxide solution and hydrogen. the lone outer shell electrons leads the alkali metal elements to share several common properties: Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). the alkali metals are a group of elements in the periodic table with similar properties: Cation formation is. Special Properties Of Alkali Metals.
From cekxuxwv.blob.core.windows.net
Properties And Uses Of Alkaline Earth Metals at Joe Riley blog Special Properties Of Alkali Metals In keeping with overall periodic trends,. the alkali metals are the elements located in group ia of the periodic table. The alkali metals are lithium, sodium, potassium, rubidium,. the group 1 elements are all soft, reactive metals with low melting points. The one outer electron is easily lost, forming the. general properties of the alkali metals. They. Special Properties Of Alkali Metals.
From slideplayer.com
Alkali Metals Electrostructure and reactivity Physical properties ppt Special Properties Of Alkali Metals general properties of the alkali metals. the alkali metals are a group of elements in the periodic table with similar properties: the group 1 elements are all soft, reactive metals with low melting points. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). Cation formation is favored by the relatively low ionization energies of. Special Properties Of Alkali Metals.
From www.youtube.com
Physical properties of alkali metals YouTube Special Properties Of Alkali Metals The alkali metals are lithium, sodium, potassium, rubidium,. general properties of the alkali metals. the alkali metals tend to form +1 cations. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The one outer electron is easily lost, forming the. Cation formation is favored by the relatively low ionization energies of the free metal (which. Special Properties Of Alkali Metals.
From cesdpdbz.blob.core.windows.net
Properties And Uses Of Alkali Metals at Nicholas Young blog Special Properties Of Alkali Metals Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The one outer electron is easily lost, forming the. the alkali metals are the elements located in group ia of the periodic table. They react with water to produce an alkaline metal hydroxide solution and hydrogen. the alkali metals tend to form +1 cations. The alkali. Special Properties Of Alkali Metals.
From ravennewsrogers.blogspot.com
Describe the Properties of Alkali Metals Special Properties Of Alkali Metals general properties of the alkali metals. the alkali metals are a group of elements in the periodic table with similar properties: Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). They react with water to produce an alkaline metal hydroxide solution and hydrogen. the group 1 elements are all soft, reactive metals with low. Special Properties Of Alkali Metals.
From ceotagpd.blob.core.windows.net
Properties Of The Alkaline Earth Metal Family at Debra Fairbanks blog Special Properties Of Alkali Metals the alkali metals tend to form +1 cations. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the. general properties of the alkali metals. the alkali metals are the elements located in group ia of the periodic table. the alkali metals are. Special Properties Of Alkali Metals.
From www.pinterest.com
Element Infographics The Alkali Metals by Compound Interest Alkali Special Properties Of Alkali Metals The one outer electron is easily lost, forming the. the lone outer shell electrons leads the alkali metal elements to share several common properties: Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). the group 1 elements are all soft, reactive metals with low melting points. They react with water to produce an alkaline metal. Special Properties Of Alkali Metals.